Organoid Models of Pancreatic Cancer
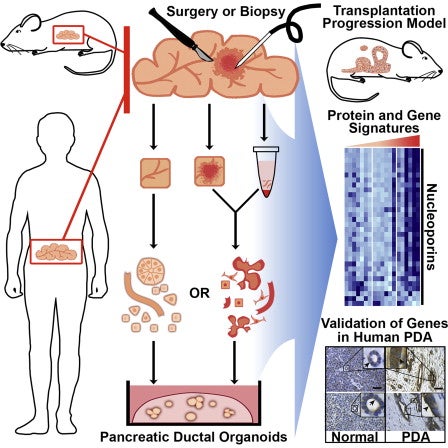
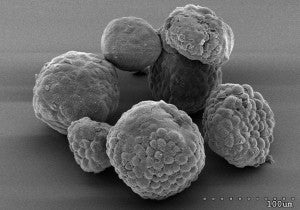
In collaboration with Hans Clevers’ Laboratory, we have developed an organoid culture system that allows us to propagate benign and malignant pancreas tissue (Boj et al. 2014). Organoids are three-dimensional cultures of cells that propagate inside of an extracellular matrix and self-assemble into structures that mimic tissue architecture in vivo. Organoids can be generated from pancreatic tumors isolated from humans or mice, and are amenable to biochemical and genetic manipulation, allowing us to probe pancreatic cancer biology in the culture dish. Our human organoids serve as a genetically diverse collection of models for studying pancreatic cancer.
We have now amassed a large and diverse collection of human pancreatic tumor organoid models from which we have generated genome and transcriptome data. We can test the efficacy of potential cancer therapies on these organoids, and identify organoids that respond or are resistant to different therapies. By comparing our genetic and transcriptome data with the results of our therapeutic testing, we are working to identify molecular signatures that correlate with treatment response. Along those lines, we recently identified a gene expression signature predictive of organoid sensitivity to chemotherapy. We are now refining this signature, and developing it for use in the clinic. We are applying similar methodology to study organoid response to targeted agents.
To determine the extent of inter-patient and intra-patient heterogeneity, we have initiated a project with multiple sites internationally to collect and characterize 300 human organoids. Single cell analyses will be performed on some of these specimens to determine the pattern of co-occurring mutations that define distinct genetic subtypes and likely represent unique therapeutic vulnerabilities. Isolation of the individual clones can be achieved as single cells can give rise to organoids.
The Pancreatic Cancer Microenvironment
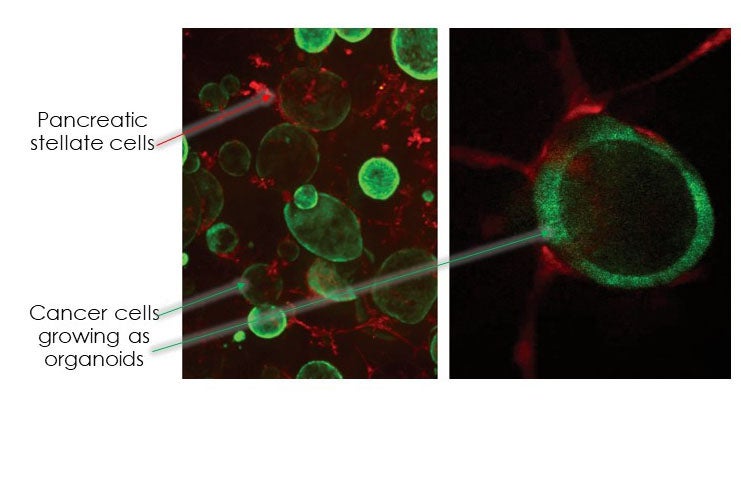
Pancreatic tumors contain high levels of desmoplastic stroma made up of extracellular matrix as well as numerous cell types. Traditionally, the stromal cells have been thought to play a tumor-supportive role. However, recent studies have challenged the dogma that stromal cells always act to benefit the pancreatic cancer cells. These studies suggest the presence of functionally distinct populations of cells within pancreatic cancer stroma. In support, we identified two distinct populations of cancer-associated fibroblasts (CAFs) in the pancreatic tumor microenvironment, a tumor-proximal, myofibroblast-like CAF population, we have termed “myCAFs,” and a tumor-distal, inflammatory CAF population we have termed “iCAFs” (Ohlund et al., 2017). Following from this work, we recently identified IL1α-JAK/STAT and TGFβ signaling cascades as the drivers of iCAF or myCAF identify, respectively (Biffi et al., 2018). Current research in the lab focuses on identifying other signaling pathways responsible for the formation and maintenance of these populations, and on identifying other novel fibroblast populations in the tumor microenvironment. We are also studying signaling between immune cells present in the tumor microenvironment and pancreatic cancer cells to better understand the pathways involved in cancer-immune crosstalk.
Redox and Metabolic Changes Associated with Pancreatic Cancer
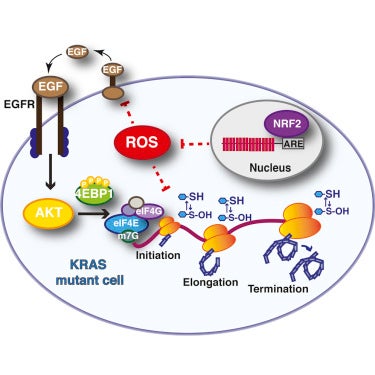
Reduction-oxidation (Redox) chemical reactions drive essential functions in all living cells, including reactions vital to cellular metabolism. Research from numerous labs has provided evidence for metabolic and redox changes associated with tumorigenesis, however the full extent of these changes in the context of pancreatic tumorigenesis remains to be defined. In addition, there is ongoing debate as to whether reactive oxygen species (ROS), oxidating agents that can act as a source for redox stress, promote or suppress tumors. ROS on the one hand, can suppress cell growth through genotoxic stress and protein translational arrest, and on the other hand, can promote cell growth through activation of mitogenic signaling cascades. Cellular responses to ROS reflect a complex integration of ROS type, location and levels. This presents a conundrum on how we should approach ROS therapy in cancer.
We study the regulation of ROS by an antioxidant transcription factor called NRF2. We previously found that levels of NRF2 are elevated during pancreatic tumorigenesis, suppressing oncogene-induced ROS. Recently, we demonstrated that genetic ablation of NRF2 led to elevated levels of hydrogen peroxide, one type of ROS, which consequently promoted oxidative inactivation of various components of the translation machinery (Chio et al., 2016). Currently, we are working to identify other cellular pathways regulated by the NRF2 antioxidant program, including regulation of redox metabolism. More broadly, we are investigating changes in cellular metabolism and mitochondrial dynamics associated with pancreatic tumorigenesis and metastatic progression.